Introduction
The history of thermodynamics as a scientific discipline generally begins with Otto von Guericke who in 1650, built and designed the world's first vacuum pump and demonstrated a vacuum using his Magdeburg hemispheres.
Heat was not formally recognized as a form of energy until about 1798, when Count Rumford (Sir Benjamin Thompson), a British military engineer, noticed that limitless amounts of heat could be generated in the boring of cannon barrels and that the amount of heat generated is proportional to the work done in turning a blunt boring tool.

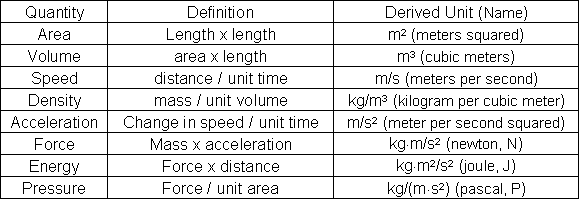
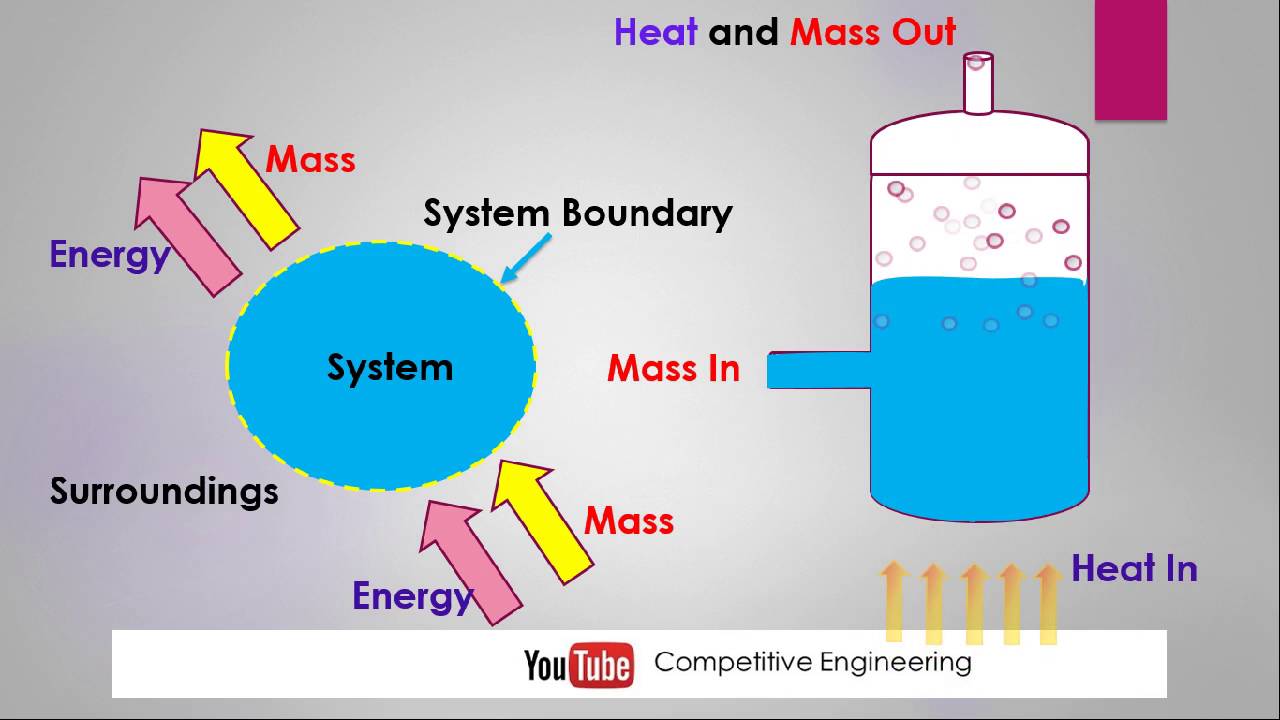
A description of any thermodynamic system employs the four laws of thermodynamics that form an axiomatic basis.
The first law specifies that energy can be exchanged between physical systems as heat and work.
The second law defines the existence of a quantity called entropy, that describes the direction, thermodynamically, that a system can evolve and quantifies the state of order of a system and that can be used to quantify the useful work that can be extracted from the system.
The third law is properties can be combined to express internal energy and thermodynamic potentials, which are useful for determining conditions for equilibrium and spontaneous processes.
The fourth law is zeroth law of thermodynamics If two systems are each in thermal equilibrium with a third system, they are in thermal equilibrium with each other. This law helps define the concept of temperature.
HistoryThe history of thermodynamics as a scientific discipline generally begins with Otto von Guericke who in 1650, built and designed the world's first vacuum pump and demonstrated a vacuum using his Magdeburg hemispheres.
Heat was not formally recognized as a form of energy until about 1798, when Count Rumford (Sir Benjamin Thompson), a British military engineer, noticed that limitless amounts of heat could be generated in the boring of cannon barrels and that the amount of heat generated is proportional to the work done in turning a blunt boring tool.



No comments:
Post a Comment